About the research topic
Purpose of the project was to integrate 3D anatomical information from CTA in fluoroscopic images in order to visualize the occluded segment from CTA, including additional relevant information such as calcifications. To this end we developed and evaluated techniques for image-based tracking of structures, focusing on instruments such as catheters and guide wires as landmarks, as well as modeling the anatomy of the patient.
Output
Layer Separation for Vessel Enhancement in Interventional X-ray Angiograms Using Morphological Filtering and Robust PCA, Ma et al., MICCAI 2015 Automatic vessel extraction from X-ray angiograms (XA) for percutaneous coronary interventions is often hampered by low contrast and presence of background structures, e.g. diaphragm, guiding catheters, stitches. In this paper, we present a novel layer separation technique for vessel enhancement in XA to address this problem. The method uses morphological filtering and Robust PCA to separate interventional XA images into three layers, i.e. a large-scale breathing structure layer, a quasi-static background layer and a layer containing the vessel structures that could potentially improve the quality of vessel extraction from XA. The method is evaluated on several clinical XA sequences. The result shows that the proposed method significantly increases the visibility of vessels in XA and outperforms other background-removal methods.
PCA-derived respiratory motion surrogates from X-ray angiograms for percutaneous coronary interventions, Ma et al., IJCARS 2015 We propose a fast automatic method for extracting patient-specific respiratory motion surrogate from cardiac XA. The method starts with an image preprocessing step to remove all tubular and curvilinear structures from XA images, such as vessels and guiding catheters, followed by principal component analysis on pixel intensities. The respiratory motion surrogate of an XA image is then obtained by projecting its vessel-removed image onto the first principal component.
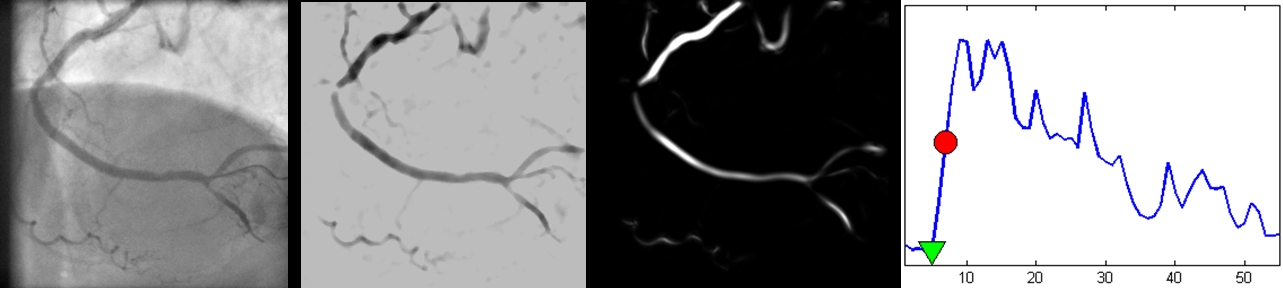
Automatic online layer separation for vessel enhancement in X-ray angiograms for percutaneous coronary interventions, Ma et al., MICCAI 2017 Automatic detection of contrast inflow in X-ray angiographic sequences can facilitate image guidance in computer-assisted cardiac interventions. In this paper, we propose two different approaches for prospective contrast inflow detection. The methods were developed and evaluated to detect contrast frames from X-ray sequences. The first approach trains a convolutional neural network (CNN) to distinguish whether a frame has contrast agent or not. The second method extracts contrast features from images with enhanced vessel structures; the contrast frames are then detected based on changes in the feature curve using long short-term memory (LSTM), a recurrent neural network architecture. Our experiments show that both approaches achieve good performance on detection of the beginning contrast frame from X-ray sequences and are more robust than a state-of-the-art method. As the proposed methods work in prospective settings and run fast, they have the potential of being used in clinical practice.
Automatic online layer separation for vessel enhancement in X-ray angiograms for percutaneous coronary interventions, Ma et al., MedIA 2017 Percutaneous coronary intervention is a minimally invasive procedure that is usually performed under image guidance using X-ray angiograms in which coronary arteries are opacified with contrast agent. In X-ray images, 3D objects are projected on a 2D plane, generating semi-transparent layers that overlap each other. The overlapping of structures makes robust automatic information processing of the X-ray images, such as vessel extraction which is highly relevant to support smart image guidance, challenging. In this paper, we propose an automatic online layer separation approach that robustly separates interventional X-ray angiograms into three layers: a breathing layer, a quasi-static layer and a vessel layer that contains information of coronary arteries and medical instruments. The method uses morphological closing and an online robust PCA algorithm to separate the three layers. The proposed layer separation method ran fast and was demonstrated to significantly improve the vessel visibility in clinical X-ray images and showed better performance than other related online or prospective approaches. The potential of the proposed approach was demonstrated by enhancing contrast of vessels in X-ray images with low vessel contrast, which would facilitate the use of reduced amount of contrast agent to prevent contrastinduced side effects.
Dynamic coronary roadmapping via catheter tip tracking in X-ray fluoroscopy with deep learning based Bayesian filtering, Ma et al., MedIA 2020 Percutaneous coronary intervention (PCI) is typically performed with image guidance using X-ray angiograms in which coronary arteries are opacified with X-ray opaque contrast agents. Interventional cardiologists typically navigate instruments using non-contrast-enhanced fluoroscopic images, since higher use of contrast agents increases the risk of kidney failure. When using fluoroscopic images, the interventional cardiologist needs to rely on a mental anatomical reconstruction. This paper reports on the development of a novel dynamic coronary roadmapping approach for improving visual feedback and reducing contrast use during PCI. The approach compensates cardiac and respiratory induced vessel motion by ECG alignment and catheter tip tracking in X-ray fluoroscopy, respectively. In particular, for accurate and robust tracking of the catheter tip, we proposed a new deep learning based Bayesian filtering method that integrates the detection outcome of a convolutional neural network and the motion estimation between frames using a particle filtering framework. The proposed roadmapping and tracking approaches were validated on clinical X-ray images, achieving accurate performance on both catheter tip tracking and dynamic coronary roadmapping experiments. In addition, our approach runs in real-time on a computer with a single GPU and has the potential to be integrated into the clinical workflow of PCI procedures, providing cardiologists with visual guidance during interventions without the need of extra use of contrast agent.


