About the research topic
The purpose of this project is to improve image guidance in 2D X-ray guided sequence abdominal catheterization procedures, specifically during transcatheter arterial chemoembolization (TACE) procedures, by using 3D information from peri/pre-operative images. During the procedure, the physician injects chemother- apeutic agents and embolizes liver tumors by inserting a catheter into the femoral artery and guiding it toward the tumors. The physician uses single-plane 2D X-ray images in which only the catheter and the ribs are visible. Pre- operative 3D Computed Tomography Angiography (CTA) and intra-operative 2D angiographies (X-rays with contrast agent) are acquired, providing detailed images of the arterial tree, and enabling a roadmap to guide the catheter. How- ever, such static roadmaps are hampered by breathing motion and catheter de- formation. We therefore propose a 3D tracking method that follows the position of the catheter tip in the 3D vessel tree, enabling guidance in the 3D image as well as facilitating continuous roadmapping.
The novel approach towards image guidance in TACE that has been developed in this project consists of:
- 2D/3D registration of an 3D vascular model (arterial tree) to 2D fluoroscopic images
- catheter tracking in 2D
- catheter tip tracking in 3D, based on 2D images and a 3D model
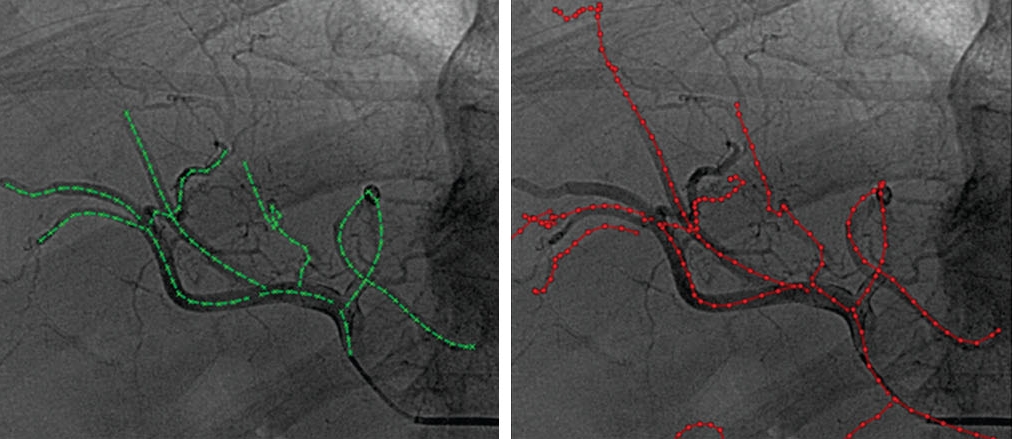 Ambrosini et al., IJCARS 2015 :
Fusion of pre/perioperative images and intraoperative
images may add relevant information during
image-guided procedures. In abdominal procedures, respiratory
motion changes the position of organs, and thus accurate
image guidance requires a continuous update of the spatial
alignment of the (pre/perioperative) information with the
organ position during the intervention.
In this study, we propose a method to register
in real time perioperative 3D rotational angiography
images (3DRA) to intra-operative single-plane 2D fluoroscopic
images for improved guidance in TACE interventions.
The method uses the shape of 3D vessels extracted from the
3DRA and the 2D catheter shape extracted from fluoroscopy.
First, the appropriate 3D vessel is selected from the complete
vascular tree using a shape similarity metric. Subsequently,
the catheter is registered to this vessel, and the 3DRA is
visualized based on the registration results. The method is
evaluated on simulated data and clinical data.
Results The first selected vessel, ranked with the shape similarity
metric, is used more than 39% in the final registration
and the second more than 21%. The median of the closest
corresponding points distance between 2D angiography vessels
and projected 3D vessels is 4.7–5.4mm when using the
brute force optimizer and 5.2–6.6mmwhen using the Powell
optimizer.
Ambrosini et al., IJCARS 2015 :
Fusion of pre/perioperative images and intraoperative
images may add relevant information during
image-guided procedures. In abdominal procedures, respiratory
motion changes the position of organs, and thus accurate
image guidance requires a continuous update of the spatial
alignment of the (pre/perioperative) information with the
organ position during the intervention.
In this study, we propose a method to register
in real time perioperative 3D rotational angiography
images (3DRA) to intra-operative single-plane 2D fluoroscopic
images for improved guidance in TACE interventions.
The method uses the shape of 3D vessels extracted from the
3DRA and the 2D catheter shape extracted from fluoroscopy.
First, the appropriate 3D vessel is selected from the complete
vascular tree using a shape similarity metric. Subsequently,
the catheter is registered to this vessel, and the 3DRA is
visualized based on the registration results. The method is
evaluated on simulated data and clinical data.
Results The first selected vessel, ranked with the shape similarity
metric, is used more than 39% in the final registration
and the second more than 21%. The median of the closest
corresponding points distance between 2D angiography vessels
and projected 3D vessels is 4.7–5.4mm when using the
brute force optimizer and 5.2–6.6mmwhen using the Powell
optimizer.
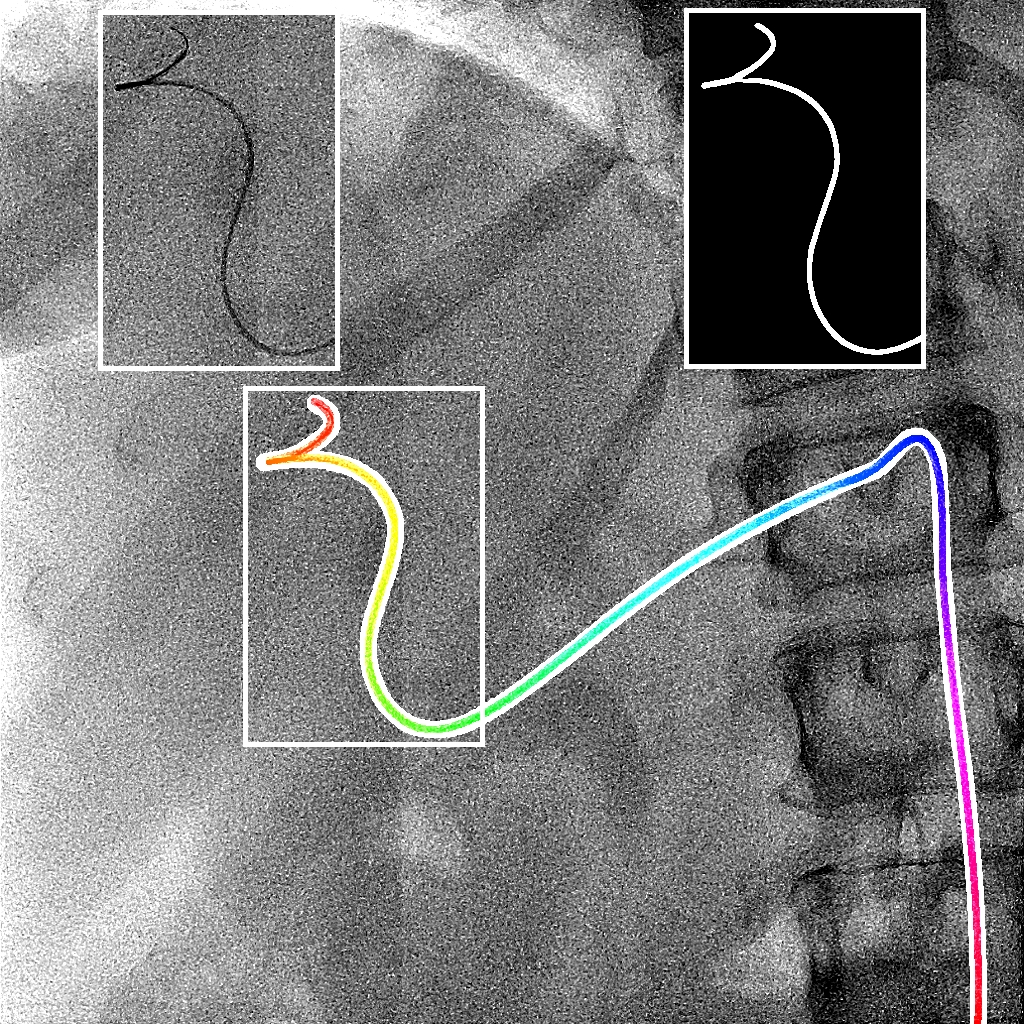 Ambrosini et al., MICCAI 2017 :
Augmenting X-ray imaging with 3D roadmap to improve
guidance is a common strategy. Such approaches benefit from automated
analysis of the X-ray images, such as the automatic detection and track-
ing of instruments. In this paper, we propose a real-time method to
segment the catheter and guidewire in 2D X-ray
uoroscopic sequences.
The method is based on deep convolutional neural networks. The net-
work takes as input the current image and the three previous ones, and
segments the catheter and guidewire in the current image. Subsequently,
a centerline model of the catheter is constructed from the segmented
image. A small set of annotated data combined with data augmentation
is used to train the network. We trained the method on images from
182 X-ray sequences from 23 different interventions. On a testing set
with images of 55 X-ray sequences from 5 other interventions, a median
centerline distance error of 0.2 mm and a median tip distance error of
0.9 mm was obtained. The segmentation of the instruments in 2D X-ray
sequences is performed in a real-time fully-automatic manner.
Ambrosini et al., MICCAI 2017 :
Augmenting X-ray imaging with 3D roadmap to improve
guidance is a common strategy. Such approaches benefit from automated
analysis of the X-ray images, such as the automatic detection and track-
ing of instruments. In this paper, we propose a real-time method to
segment the catheter and guidewire in 2D X-ray
uoroscopic sequences.
The method is based on deep convolutional neural networks. The net-
work takes as input the current image and the three previous ones, and
segments the catheter and guidewire in the current image. Subsequently,
a centerline model of the catheter is constructed from the segmented
image. A small set of annotated data combined with data augmentation
is used to train the network. We trained the method on images from
182 X-ray sequences from 23 different interventions. On a testing set
with images of 55 X-ray sequences from 5 other interventions, a median
centerline distance error of 0.2 mm and a median tip distance error of
0.9 mm was obtained. The segmentation of the instruments in 2D X-ray
sequences is performed in a real-time fully-automatic manner.
![]() Ambrosini et al., TMI 2017 :
In minimal invasive image guided catheterization
procedures, physicians require information of the
catheter position with respect to the patient’s vasculature.
However, in fluoroscopic images, visualization of the vasculature
requires toxic contrast agent. Static vasculature
roadmapping, which can reduce the usage of iodine contrast,
is hampered by the breathing motion in abdominal
catheterization. In this paper, we propose a method to track
the catheter tip inside the patient’s 3D vessel tree using
intra-operative single-plane 2D X-ray image sequences and
a peri-operative 3D rotational angiography (3DRA). The
method is based on a hidden Markov model (HMM) where
states of themodel are the possible positions of the catheter
tip inside the 3D vessel tree. The transitions from state to
state model the probabilities for the catheter tip to move
from one position to another. The HMM is updated following
the observation scores, based on the registration between
the 2D catheter centerline extracted from the 2D X-ray
image, and the 2D projection of 3D vessel tree centerline
extracted from the 3DRA. The method is extensively evaluated
on simulated and clinical datasets acquired during liver
abdominal catheterization. The evaluations show a median
3D tip tracking error of 2.3 mm with optimal settings in
simulated data. The registered vessels close to the tip have
a median distance error of 4.7 mm with angiographic data
and optimal settings. Such accuracy is sufficient to help
the physicianswith an up-to-date roadmapping.The method
tracks in real-time the catheter tip and enables roadmapping
during catheterization procedures.
Ambrosini et al., TMI 2017 :
In minimal invasive image guided catheterization
procedures, physicians require information of the
catheter position with respect to the patient’s vasculature.
However, in fluoroscopic images, visualization of the vasculature
requires toxic contrast agent. Static vasculature
roadmapping, which can reduce the usage of iodine contrast,
is hampered by the breathing motion in abdominal
catheterization. In this paper, we propose a method to track
the catheter tip inside the patient’s 3D vessel tree using
intra-operative single-plane 2D X-ray image sequences and
a peri-operative 3D rotational angiography (3DRA). The
method is based on a hidden Markov model (HMM) where
states of themodel are the possible positions of the catheter
tip inside the 3D vessel tree. The transitions from state to
state model the probabilities for the catheter tip to move
from one position to another. The HMM is updated following
the observation scores, based on the registration between
the 2D catheter centerline extracted from the 2D X-ray
image, and the 2D projection of 3D vessel tree centerline
extracted from the 3DRA. The method is extensively evaluated
on simulated and clinical datasets acquired during liver
abdominal catheterization. The evaluations show a median
3D tip tracking error of 2.3 mm with optimal settings in
simulated data. The registered vessels close to the tip have
a median distance error of 4.7 mm with angiographic data
and optimal settings. Such accuracy is sufficient to help
the physicianswith an up-to-date roadmapping.The method
tracks in real-time the catheter tip and enables roadmapping
during catheterization procedures.
Funding: Philips

