About the research topic
Soft tissue tumors (STT) are a rare and complex group of lesions with a broad range of differentiation. All STT subtypes greatly differ in their clinical behavior, aggressiveness, molecular background, and preferred treatments given. Diagnosis of the correct phenotype, the grade of aggressiveness, and molecular make-up is therefore of utmost importance. Diagnosis of STT is generally supported by imaging, such as computed tomography (CT) and magnetic resonance imaging (MRI). However, visual assessment by a radiologist tends to be subjective and not precise. Quantitative, computational (“radiomics”) imaging features and state-of-the-art Artificial Intelligence (AI) techniques based on machine learning could enable more objective and precise STT diagnosis. With the support of the Hanarth Foundation, we develop a comprehensive STT diagnostic model, both for phenotyping and grading, based on quantitative image analysis by radiomics and deep learning. Our AI model will guide diagnosis and treatment decisions, thereby facilitating personalized medicine.
In order to train and validate this model we have setup a large, multi-center cohort. This Sarcoma Artificial Intelligence (SAI) consortium is a collaboration between various sarcoma expert centers in Europe and the United States.
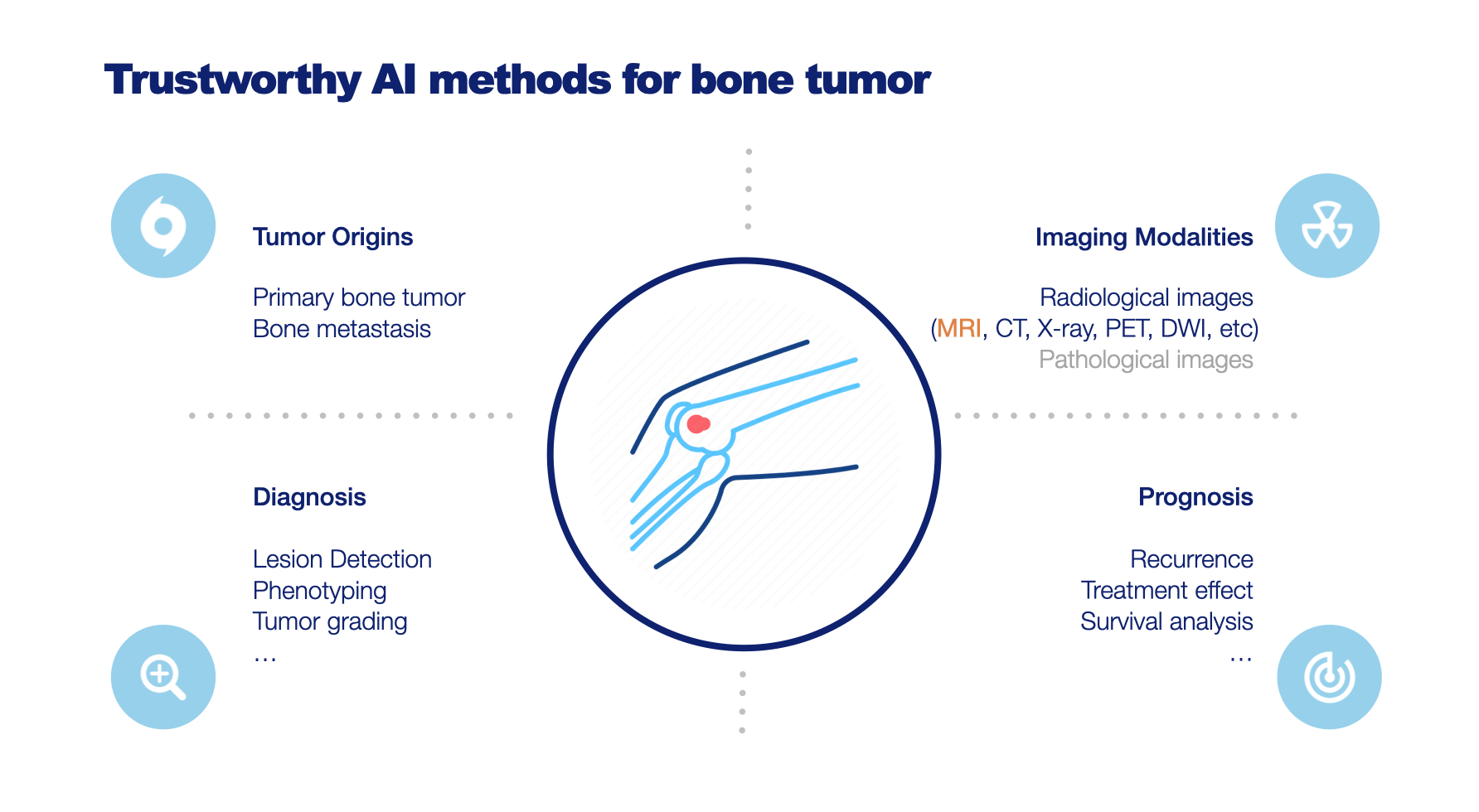
Develop Trustworthy AI methods for Improved Diagnosis of Bone and Soft-tissue Lesions on MRI
Magnetic Resonance Imaging (MRI) plays a pivotal role in the differential diagnosis and staging of bone tumors, soft-tissue tumors, and tumor-like lesions. However, the wide variety of lesions, their shared clinical manifestations, and the need for considering multiple imaging modalities make image interpretation a highly challenging and specialized task. To address this, we propose the implementation of AI-based image analysis algorithms to aid radiologists in predicting the bone tumor type or narrowing down the subset of most likely tumor types, thereby enhancing the differential diagnosis process. Additionally, we will prioritize the trustworthiness of these AI models by employing explainable AI techniques to ensure reliable and interpretable results. Furthermore, we compare different imaging modalities and provide suggestions for their use in establishing a more definitive diagnosis, predicting treatment response, streamlining the imaging process, and enhancing overall efficiency. The successful implementation of these AI models has the potential to improve diagnostic accuracy, revolutionize musculoskeletal imaging, and ultimately benefit patient management and outcomes. This project is under Trustworthy AI for MRI Lab, which is part of the ROBUST program on Trustworthy AI-based Systems for Sustainable Growth under the NWO LTP funding scheme.